Advancing Cancer Treatment in Alignment with Sustainable Development Goal 3
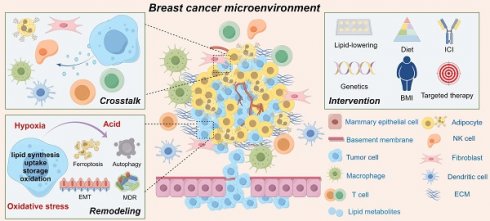
In the global effort to achieve Sustainable Development Goal 3 (SDG 3), which aims to ensure good health and well-being for all, a primary objective is Target 3.4: to reduce premature mortality from non-communicable diseases, including cancer. Research into optimizing cancer treatments is fundamental to this goal. This report details findings on the use of radiotherapy (RT) to modify the tumor microenvironment (TME) in breast cancer, a strategy aimed at improving the efficacy of immunotherapy and contributing to more effective cancer care worldwide.
Analysis of Radiotherapy’s Immunological Impact on Breast Cancer
Background and Rationale
The efficacy of immunotherapy, particularly with immune checkpoint inhibitors (ICIs), is limited in patients with HR+HER2– breast cancer, a subtype often characterized as immunologically “silent.” Focal RT has been proposed as a method to convert these tumors into immunologically active environments that are more responsive to ICIs. However, the immunological outcome of RT is highly dependent on variables such as dose and fractionation schedule. Recent findings from a study by Schalck and colleagues provide critical insights, corroborating that the RT dose is a decisive factor in modulating the TME, with significant implications for achieving SDG 3 through improved treatment protocols.
Clinical Investigation and Methodology
The research centered on the PRECISE Phase II clinical trial, which investigated the effects of neoadjuvant focal RT on women with HR+HER2– breast cancer. The study’s design and methods are outlined below:
- Patient Cohort: 19 women with HR+HER2– breast cancer.
- Intervention: Neoadjuvant focal RT delivered prior to surgery.
- Dosage Regimens: Two biologically equivalent boost doses were administered: a single 7.5 Gy fraction or five 2 Gy fractions.
- Primary Assessment: Longitudinal analysis of tumor-infiltrating lymphocytes (TILs).
- Advanced Analysis: A subset of patient samples underwent single-cell (sc) DNA, RNA, and T-cell receptor (TCR) sequencing to map genomic and immunological changes.
Key Findings on the Tumor Microenvironment (TME)
Effects on T-Cell Populations
Longitudinal analysis revealed complex and sometimes contradictory effects of focal RT on T-cell populations within the TME. The key observations include:
- A marginal increase in TILs was noted in 11 of 19 patients, though this change was not statistically significant between the two RT schedules.
- Surgical specimens post-RT were enriched in naïve or central-memory CD4+ T cells, without a parallel increase in regulatory T (TREG) cells.
- A general decrease was observed in lymphocytes expressing immune effectors from the granzyme family.
- Notably, one specific population of CD4+ T cells expressing crucial anticancer cytokines, such as TNF and IFNG, was identified.
- TCR analysis indicated that RT substantially reconfigured the T-cell infiltrate, suggesting it eliminated existing TILs while promoting the recruitment of new clonotypes.
Alterations in Myeloid Cells
Tumor-infiltrating myeloid cells also underwent significant changes following RT, with findings suggesting a shift towards an immunosuppressive phenotype, a critical consideration for therapeutic strategies aligned with SDG 3.
- The relative abundance of tumor-associated macrophages (TAMs) increased post-irradiation.
- Specifically, there was a relative increase in TAMs expressing FCGBP and CX3CR1. This phenotype has been previously associated with clinically relevant immunosuppression and therapy resistance.
- In a potentially favorable observation, myeloid cells as a whole expressed higher levels of genes related to antigen presentation, which could support T-cell activation.
- However, the abundance of dendritic cells (DCs), which are critical for initiating robust anticancer immunity, showed only marginal alterations.
Impact on Malignant Cells and Genomic Selection
The study found that RT did not induce widespread transcriptional changes in malignant cells that outweighed patient-specific signatures. However, specific alterations and differential responses were identified.
- Key genes involved in type I interferon (IFN) signaling were surprisingly downregulated post-RT, an outcome generally considered unfavorable for antitumor immunity.
- Longitudinal scDNAseq analysis revealed that RT altered the subclonal composition of malignant cells, allowing for the stratification of patients into High Genomic Selection (HGS) and Low Genomic Selection (LGS) cohorts.
- The LGS cohort was enriched for IFN-related transcripts, including a signature associated with radioresistance. These samples also had a higher proportion of immune cells post-RT but were simultaneously enriched in transcripts associated with active immunosuppression, pointing to an ongoing but ineffective immune response.
Report Conclusions and Implications for SDG 3
The Critical Role of Radiotherapy Dose
The primary conclusion of this report is that the boost dose of focal RT used in the trial (biologically equivalent to 7.5 Gy in a single fraction) predominantly elicited immunological changes associated with an immunosuppressive TME. This was most evident in the increase of myeloid cells and the predominance of CXC3CR1+ TAMs. This outcome contrasts sharply with preclinical evidence suggesting that higher, hypofractionated RT doses (e.g., total doses of 24–50 Gy) can successfully convert immunologically silent tumors into active ones responsive to ICIs. These findings underscore that dose and fractionation are critical determinants of RT’s biological effects, a crucial piece of knowledge for designing effective combination therapies.
Contribution to Global Health Goals
This research directly supports the ambitions of **SDG 3: Good Health and Well-being**. By elucidating the dose-dependent immunological effects of RT, these findings contribute to the essential knowledge base required to refine and personalize cancer treatments. Optimizing therapeutic protocols to avoid immunosuppressive outcomes and instead promote robust anti-tumor immunity is vital for improving patient survival and quality of life. This work highlights the complexity of cancer therapy and reinforces the need for continued research and development, as called for in **SDG Target 3.b**, to create more effective strategies that will help reduce premature mortality from non-communicable diseases and realize the global vision of health for all.
Which SDGs are addressed or connected to the issues highlighted in the article?
-
SDG 3: Good Health and Well-being
The article is fundamentally centered on improving health outcomes for a specific disease. It discusses advanced research into breast cancer, a major non-communicable disease. The entire focus is on understanding how to make treatments like immunotherapy more effective for patients with a specific subtype of breast cancer (HR+HER2–). The research aims to “convert immunologically silent HR+ breast malignancies into immunologically active tumors that may respond to ICIs,” directly contributing to the goal of ensuring healthy lives and promoting well-being.
-
SDG 9: Industry, Innovation, and Infrastructure
The article showcases cutting-edge scientific research and technological innovation in the medical field. It details the use of advanced techniques such as “focal radiation therapy (RT),” “single-cell (sc) DNA, RNA, and TCR sequencing (seq),” and “immunohistochemistry (IHC).” This work, conducted through a “Phase II clinical trial (PRECISE, NCT03359954),” represents a significant effort in scientific research and innovation aimed at upgrading technological capabilities in healthcare to solve complex medical challenges.
-
SDG 17: Partnerships for the Goals
The research presented in the article is a clear example of multi-stakeholder partnerships. The acknowledgements section details a wide range of collaborations essential for this type of advanced research. It lists support from public institutions (“NIH R01 grant,” “US DoD BCRP”), non-profit foundations (“William Guy Forbeck Research Foundation,” “Stand Up to Cancer (SU2C)”), and “industrial collaborations with Lytix Biopharma… Promontory… and Onxeo.” This demonstrates a partnership model that mobilizes financial resources, technology, and knowledge from public, private, and civil society sectors to achieve a common health goal.
What specific targets under those SDGs can be identified based on the article’s content?
SDG 3: Good Health and Well-being
-
Target 3.4: By 2030, reduce by one-third premature mortality from non-communicable diseases through prevention and treatment and promote mental health and well-being.
The article directly addresses this target by focusing on improving treatment for breast cancer, a leading non-communicable disease. The research into combining radiotherapy and immunotherapy seeks to enhance treatment efficacy for “the majority of women with breast neoplasms” who have a subtype that is “poorly responsive to ICIs,” thereby contributing to reducing premature mortality from this disease.
-
Target 3.b: Support the research and development of vaccines and medicines for the communicable and non-communicable diseases that primarily affect developing countries, provide access to affordable essential medicines and vaccines…
The article is a direct report on the “research and development” of new therapeutic strategies for a non-communicable disease. It describes a “Phase II clinical trial” and “preclinical evidence” aimed at developing more effective treatments, which is the core activity of this target.
SDG 9: Industry, Innovation, and Infrastructure
-
Target 9.5: Enhance scientific research, upgrade the technological capabilities of industrial sectors in all countries… including… encouraging innovation and substantially increasing the number of research and development workers… and public and private research and development spending.
The article is an outcome of enhanced scientific research. The authors are R&D workers, and the acknowledgements section explicitly lists numerous sources of “public and private research and development spending” (e.g., “NIH R01 grant,” “US DoD BCRP,” “industrial collaborations”) that fund this innovation.
SDG 17: Partnerships for the Goals
-
Target 17.17: Encourage and promote effective public, public-private and civil society partnerships, building on the experience and resourcing strategies of partnerships.
The funding and collaboration model described in the acknowledgements section is a textbook example of this target in action. It lists partnerships between academic research centers (“Fox Chase Cancer Center”), public funding bodies (“NIH,” “US DoD”), private industry (“Lytix Biopharma,” “Onxeo”), and non-profit foundations (“Stand Up to Cancer”), showcasing an effective multi-stakeholder partnership to advance medical science.
Are there any indicators mentioned or implied in the article that can be used to measure progress towards the identified targets?
SDG 3: Good Health and Well-being
- Implied progress towards Indicator 3.4.1: (Mortality rate attributed to… cancer). While the article does not provide mortality data, its primary goal of improving cancer treatment directly contributes to the reduction of this rate.
-
Proxy Indicators mentioned in the article: The study uses several specific, measurable biological markers to assess treatment effectiveness, which serve as proxies for progress. These include:
- “longitudinal quantification of tumor-infiltrating lymphocytes (TILs)”
- “relative abundance of tumor-associated macrophages (TAMs)”
- “IHC-assisted quantification of the marker of proliferation Ki-67”
- Changes in “TCR clonotypes” to reconfigure the T cell infiltrate.
- Assessment of “pathological complete responses” in patients.
SDG 9: Industry, Innovation, and Infrastructure
- Implied data for Indicator 9.5.1: (Research and development expenditure as a proportion of GDP). The article explicitly mentions numerous grants and funding sources (“one NIH R01 grant (#CA271915)”, “two Breakthrough Level 2 grants from the US DoD BCRP”, “a grant from the STARR Cancer Consortium”), which represent the R&D expenditure being measured by this indicator.
- Implied data for Indicator 9.5.2: (Researchers… per million inhabitants). The authors of the paper (Lorenzo Galluzzi, Lukas Bolini, Rebecca M. Shulman) and the colleagues they cite are the researchers being counted in this indicator.
SDG 17: Partnerships for the Goals
- Evidence for Indicator 17.17.1: (Amount of United States dollars committed to public-private and civil society partnerships). The article provides direct evidence of these partnerships by naming the specific public (“NIH”, “DoD”), private (“Lytix Biopharma”, “Promontory”), and civil society (“Stand Up to Cancer”) organizations providing financial and collaborative support for the research.
SDGs, Targets, and Indicators Analysis
| SDGs | Targets | Indicators (Mentioned or Implied in the Article) |
|---|---|---|
| SDG 3: Good Health and Well-being | 3.4: Reduce premature mortality from non-communicable diseases (NCDs) through prevention and treatment. | The research aims to improve treatment for breast cancer (an NCD). Progress is measured by proxy indicators like “quantification of tumor-infiltrating lymphocytes (TILs),” changes in “tumor-associated macrophages (TAMs),” and achieving “pathological complete responses.” |
| 3.b: Support the research and development of medicines for NCDs. | The article describes a “Phase II clinical trial (PRECISE, NCT03359954)” and “preclinical evidence” for new radiotherapy/immunotherapy combinations. | |
| SDG 9: Industry, Innovation, and Infrastructure | 9.5: Enhance scientific research and encourage innovation. | The article is a product of scientific research funded by R&D spending (“NIH R01 grant,” “US DoD BCRP grants”). It utilizes innovative technologies like “single-cell (sc) DNA, RNA, and TCR sequencing.” |
| SDG 17: Partnerships for the Goals | 17.17: Encourage and promote effective public, public-private and civil society partnerships. | The acknowledgements section lists specific public (“NIH”), private (“Onxeo,” “Lytix Biopharma”), and civil society (“Stand Up to Cancer”) partners, providing direct evidence of these collaborations. |
Source: nature.com