Report on the Post-Marketing Safety Evaluation of Etrasimod for Ulcerative Colitis in the Context of Sustainable Development Goals
Executive Summary
This report presents a comprehensive, real-world safety evaluation of etrasimod, a sphingosine 1-phosphate (S1P) receptor modulator used for treating ulcerative colitis (UC). In alignment with Sustainable Development Goal 3 (SDG 3), which aims to ensure healthy lives and promote well-being for all, this study utilizes data from the FDA Adverse Event Reporting System (FAERS) to enhance patient safety and support the responsible use of innovative medicines. A disproportionality analysis was conducted on all adverse events (AEs) reported since 2004, identifying significant safety signals across multiple system organ classes (SOCs). Key findings include previously unreported AEs such as headache, dizziness, fatigue, and blurred vision. This research provides critical real-world evidence that empowers clinicians to safeguard patient health, contributing directly to the global objective of achieving safe and effective healthcare for non-communicable diseases.
Introduction: Aligning Pharmaceutical Safety with Sustainable Development Goals
The management of chronic inflammatory diseases like ulcerative colitis (UC) is a critical public health challenge. The development of novel therapeutics such as etrasimod represents significant progress in medical innovation. However, ensuring the safety of these new treatments is paramount to achieving global health targets.
SDG 3: Ensuring Healthy Lives and Promoting Well-being
Sustainable Development Goal 3 (SDG 3) emphasizes the need to reduce premature mortality from non-communicable diseases and ensure access to safe, effective, and quality medicines. Ulcerative colitis, affecting an estimated 5 million people globally, diminishes quality of life and increases the risk of colorectal cancer. Effective and safe treatment is essential for managing this condition. Post-marketing surveillance of drugs like etrasimod is a fundamental component of a robust healthcare system, directly contributing to the targets of SDG 3 by providing the evidence needed for safe clinical practice.
Context of Ulcerative Colitis and Etrasimod
Etrasimod is a novel, oral S1P receptor modulator approved for adults with moderate to severe active UC. It functions as a selective immunomodulator by reducing pro-inflammatory immune cells. While clinical trials have established its efficacy and short-term safety, a comprehensive evaluation of its real-world safety profile is necessary to guide long-term clinical use and protect patient well-being. This study addresses this gap by analyzing post-marketing adverse drug events (ADEs) from the FAERS database.
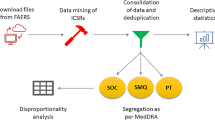
Methodology: A Data-Driven Approach to Pharmacovigilance
This study employed a rigorous, data-driven methodology to analyze the safety of etrasimod, leveraging public health infrastructure in line with SDG 9 (Industry, Innovation, and Infrastructure) and collaborative data sharing principles central to SDG 17 (Partnerships for the Goals).
Data Source and Extraction
- Data Source: Publicly available raw data were sourced from the U.S. FDA Adverse Event Reporting System (FAERS) database, covering the period from the first quarter of 2004 to 2024.
- Data Extraction: The search term ‘etrasimod’ was used to extract all relevant records from the seven FAERS data files, including demographic, drug, indication, and adverse event information.
Data Processing and Analysis
- Deduplication: Duplicate reports were removed using the FDA-recommended method, resulting in a final dataset of 18,613,992 unique demographic records.
- Screening: Reports were screened to identify cases where etrasimod was the primary suspect (PS) drug, yielding 1,406 AEs associated with etrasimod.
- Statistical Analysis: Disproportionality analysis was conducted to identify potential safety signals. Four standard methods were employed:
- Reporting Odds Ratio (ROR)
- Proportional Reporting Ratio (PRR)
- Multi-item Gamma Poisson Shrinkage (MGPS)
- Bayesian Confidence Propagation Neural Network (BCPNN)
- Temporal Analysis: The time to onset of AEs was modeled using a Weibull distribution to identify periods of highest risk.
Results: Real-World Safety Profile of Etrasimod
The analysis of 1,406 AE reports where etrasimod was the primary suspect drug revealed important insights into its real-world safety profile.
Demographic and Reporting Characteristics
- Gender Distribution: Females accounted for 44.65% of reports, and males for 42.23%.
- Age Distribution: The largest proportion of reports came from the 18–44 age group (36.8%), followed by the 45–64 age group (30.02%).
- Reporting Source: Consumers were the primary reporters (43.29%).
- Geographic Origin: The majority of reports originated from the United States (95.17%).
- Serious Outcomes: Among serious AEs, hospitalization was the most common (4.68%), followed by death (0.3%).
Analysis of Adverse Events by System Organ Class (SOC)
Significant positive signals were detected for etrasimod-related AEs across several SOCs, indicating a broad impact on patient health. These findings are crucial for holistic patient monitoring, a key aspect of SDG 3.
- Gastrointestinal Disorders: ROR = 2.36 (95% CI: 2.06–2.71)
- Eye Disorders: ROR = 5.18 (95% CI: 4.34–6.19)
- Metabolic and Nutritional Disorders: ROR = 3.49 (95% CI: 2.85–4.28)
- General Disorders and Administration Site Conditions: ROR = 1.59 (95% CI: 1.41–1.80)
- Immune System Disorders: ROR = 1.56 (95% CI: 1.04–2.33)
Identification of Specific Adverse Events (PT Level)
The analysis confirmed known AEs listed on the drug label and identified several potential new safety signals not currently listed.
- Confirmed AEs: Hypertension, bradycardia, dyspnea, hepatic injury, and macular edema.
- Potential Unlabeled AEs:
- Headache (ROR = 3.07)
- Dizziness (ROR = 2.86)
- Fatigue (ROR = 1.73)
- Diarrhea (ROR = 1.82)
- Blurred vision
- Abnormal blood cholesterol (ROR = 47.33)
- Hematochezia (ROR = 17.34)
Temporal Analysis of Adverse Event Onset
The Weibull distribution analysis indicated an early failure mode, with a median time to onset of AEs of 41 days. The majority of AEs occurred within the first 30 days of treatment, highlighting a critical window for patient monitoring to ensure health and well-being.
Discussion: Implications for Public Health and Sustainable Healthcare
The findings of this study provide actionable insights for clinicians and policymakers, reinforcing the importance of pharmacovigilance in achieving sustainable health outcomes.
Contribution to SDG 3: Good Health and Well-being
This research directly supports SDG 3 by generating real-world evidence on drug safety. By identifying both known and potential new AEs, the study equips healthcare providers with the information needed to mitigate risks, optimize treatment plans, and improve the quality of life for patients with UC. Early and vigilant monitoring, especially within the first month of treatment, is crucial to preventing serious outcomes and ensuring that innovative treatments contribute positively to public health.
Supporting SDG 9: Innovation and Infrastructure
The use of the FAERS database demonstrates the value of robust data infrastructure (SDG 9) for post-marketing surveillance. Such systems are essential for the sustainable integration of new medical innovations into healthcare practice. By providing a feedback loop from real-world use to clinical knowledge, this analysis ensures that innovation is paired with responsibility and safety.
Limitations and Future Directions
The study acknowledges limitations inherent to voluntary reporting systems, including potential underreporting and data incompleteness. The findings are primarily based on U.S. data, which may limit generalizability.
- Future research should aim to validate these findings with larger, more diverse datasets.
- Integrating data from other regional databases (e.g., EudraVigilance) could strengthen the robustness of the conclusions.
- Long-term prospective studies are needed to confirm the causal relationship between etrasimod and the identified AEs.
Conclusion: Enhancing Patient Safety through Evidence-Based Practice
This comprehensive analysis of the FAERS database provides critical real-world safety information for etrasimod. The identification of potential unlabeled AEs underscores the necessity of continuous post-marketing surveillance. By translating data into clinical guidance, this research contributes significantly to the principles of SDG 3, promoting safer and more effective healthcare. These findings empower clinicians to better manage treatment, optimize patient outcomes, and uphold the global commitment to ensuring healthy lives and well-being for all.
Analysis of Sustainable Development Goals in the Article
1. Which SDGs are addressed or connected to the issues highlighted in the article?
The primary Sustainable Development Goal (SDG) connected to the issues discussed in the article is:
-
SDG 3: Good Health and Well-being.
- The article’s central theme is the evaluation of the clinical safety of etrasimod, a drug used to treat ulcerative colitis (UC). This directly aligns with SDG 3’s mission to “Ensure healthy lives and promote well-being for all at all ages.” The research focuses on pharmacovigilance, which is a critical component of public health systems aimed at safeguarding patient health and improving the quality of healthcare. By providing “real-world evidence on the safety profile of etrasimod,” the study contributes to better management of a chronic non-communicable disease and ensures that treatments are safe and effective.
2. What specific targets under those SDGs can be identified based on the article’s content?
-
Target 3.4: By 2030, reduce by one third premature mortality from non-communicable diseases through prevention and treatment and promote mental health and well-being.
- The article addresses this target by focusing on ulcerative colitis, which it describes as an “idiopathic chronic inflammatory disease.” The study’s goal of ensuring the safe use of etrasimod is a direct contribution to the “treatment” aspect of managing non-communicable diseases (NCDs).
- The article explicitly links UC to diminished quality of life and mental health, stating that “Patients with UC have a high prevalence of mental disorders such as depression and anxiety.” By evaluating and improving the safety of treatments, the research aims to enhance patient outcomes and well-being.
-
Target 3.8: Achieve universal health coverage, including financial risk protection, access to quality essential health-care services and access to safe, effective, quality and affordable essential medicines and vaccines for all.
- The research is fundamentally about ensuring access to “safe, effective, quality” medicines. The abstract states the study’s purpose is a “comprehensive and real-world evaluation of its clinical safety” to provide “guidance for clinicians on safe medication use.”
- By identifying both known and “previously unreported AEs not currently listed on drug label,” the study enhances the knowledge base required to ensure that medicines provided to patients meet high safety standards, a cornerstone of quality healthcare services.
-
Target 3.d: Strengthen the capacity of all countries, in particular developing countries, for early warning, risk reduction and management of national and global health risks.
- The entire study is an application of a health risk management system. The article describes the FDA Adverse Event Reporting System (FAERS) as a database used “to monitor the safety of marketed drugs and biologics, identify potential AEs, and support FDA regulatory decisions.”
- The methodology, which involves data mining and “disproportionality analysis” of adverse event reports, exemplifies a sophisticated approach to early warning and risk reduction in pharmacology. This process allows for the continuous monitoring of drug safety after a product has been marketed, which is a key capacity for managing health risks.
3. Are there any indicators mentioned or implied in the article that can be used to measure progress towards the identified targets?
While the article does not explicitly name official SDG indicators, its data and methodology serve as practical, real-world examples of how progress towards these targets can be measured:
-
Implied Indicators for Target 3.4 (NCDs and Mental Health)
- Burden of Disease Data: The article mentions that the “global prevalence of UC is estimated to be 5 million cases annually” and that it increases the “risk of developing colorectal cancer.” This type of epidemiological data is crucial for tracking the burden of NCDs.
- Quality of Life and Mental Health Metrics: The discussion on how UC symptoms are “associated with a diminished quality of life” and the high prevalence of “depression and anxiety” in patients implies the need for indicators that measure well-being beyond simple mortality rates.
-
Implied Indicators for Target 3.8 (Access to Safe Medicines)
- Adverse Event Reporting Rates: The core of the study is the analysis of 1,406 adverse event (AE) reports. The number, type, and severity of AEs (e.g., hospitalization, disability, death) are direct measures of a medicine’s safety profile in a real-world setting.
- Statistical Safety Signals: The article uses specific statistical measures like the “reporting odds ratio (ROR)” and “proportional reporting ratio (PRR)” to identify safety signals. For example, it reports “eye disorders(ROR = 5.18, 95% CI: 4.34–6.19).” These statistical values are quantitative indicators used to assess the safety of medicines.
-
Implied Indicators for Target 3.d (Health Risk Management)
- Functionality of Pharmacovigilance Systems: The article’s use of the FAERS database demonstrates a functioning national system for health risk surveillance. The process described—from data extraction and deduplication to statistical analysis—is an indicator of a country’s capacity for post-marketing drug monitoring.
- Timeliness of AE Detection: The analysis of the “onset time of AEs,” which found that “most AEs occurred within the first 30 days of treatment,” is an indicator of the system’s ability to provide early warnings to clinicians and patients, thus strengthening risk management.
4. Summary Table of SDGs, Targets, and Indicators
| SDGs | Targets | Indicators (Implied from the Article) |
|---|---|---|
| SDG 3: Good Health and Well-being | 3.4: Reduce premature mortality from NCDs and promote mental health. |
|
| 3.8: Achieve universal health coverage, including access to safe, effective, and quality essential medicines. |
|
|
| 3.d: Strengthen capacity for early warning, risk reduction, and management of health risks. |
|
Source: nature.com