Report on the Real-World Safety Assessment of Tamsulosin and its Implications for Sustainable Development Goals
Executive Summary
This report presents a retrospective pharmacovigilance analysis of tamsulosin, an alpha-1-adrenergic receptor antagonist, utilizing data from the FDA Adverse Event Reporting System (FAERS) from 2004 to 2024. In alignment with Sustainable Development Goal 3 (Good Health and Well-being), this study evaluates the safety profile of this widely used medication to enhance patient safety and promote the effective management of non-communicable diseases. A total of 10,071 adverse event (AE) reports were analyzed. The findings revealed a concentration of AEs in the early phase of treatment and significant demographic differences in reporting patterns. Subgroup analyses highlighted distinct AE profiles based on gender and age, directly addressing SDG 5 (Gender Equality) and SDG 10 (Reduced Inequalities) by providing disaggregated health data. In addition to confirming known AEs, the analysis identified new potential safety signals, including hyperhidrosis, cystitis, panic reaction, and painful erection. These findings underscore the importance of post-marketing surveillance in achieving universal health coverage with safe and effective medicines.
Introduction: Aligning Pharmacovigilance with Sustainable Development Goals
Tamsulosin is a critical medication for managing lower urinary tract symptoms (LUTS), primarily associated with benign prostatic hyperplasia (BPH). As the global population ages, the prevalence of BPH is increasing, making the safe use of tamsulosin a key component of promoting healthy lives and well-being for an aging demographic, a core tenet of SDG 3. Ensuring access to safe, effective, and affordable essential medicines (SDG Target 3.8) requires robust post-marketing surveillance. While tamsulosin is generally considered safe, understanding its real-world adverse event (AE) profile is essential for managing non-communicable diseases effectively. This study leverages the FAERS database to conduct a systematic analysis of tamsulosin-related AEs, providing critical evidence to support global health objectives.
Methodological Framework for Sustainable Health Outcomes
Data Source and Contribution to SDG 17
This retrospective analysis utilized the FDA Adverse Event Reporting System (FAERS) database, a public resource containing spontaneous AE reports. The use of such open-access, large-scale databases exemplifies SDG 17 (Partnerships for the Goals), which encourages global partnerships and the sharing of knowledge, science, and technology to support sustainable development. Data from the first quarter of 2004 to the second quarter of 2024 were extracted for this analysis.
Study Design and Data Processing
The study was designed as a retrospective pharmacovigilance analysis. The workflow involved the following steps:
- Extraction of 21,433,114 spontaneous reports from the FAERS database.
- Exclusion of duplicate entries, resulting in 17,956,653 unique reports.
- Isolation of 10,071 case reports where tamsulosin was listed as the primary suspect (PS) drug.
- Standardization of AEs using the Medical Dictionary for Regulatory Activities (MedDRA) at the System Organ Class (SOC) and Preferred Term (PT) levels.
Statistical Analysis
To detect safety signals, four disproportionality analyses were employed. These methods are crucial for identifying potential risks that may not be apparent in clinical trials, thereby strengthening health systems in line with SDG 3.
- Proportional Reporting Ratio (PRR)
- Reporting Odds Ratio (ROR)
- Bayesian Confidence Propagation Neural Network (BCPNN)
- Empirical Bayesian Geometric Mean (EBGM)
Bonferroni correction was applied to control for type I errors. Further analyses included time-to-onset evaluation and subgroup stratification by age and gender to assess the stability of the findings.
Results: A Comprehensive Safety Profile for Tamsulosin
Baseline Demographics and Global Health Implications
The analysis of 10,071 reports revealed key demographic characteristics relevant to global health monitoring under SDG 3.
- Gender: AEs were reported more frequently in men, consistent with the primary indication for BPH.
- Age: AEs were concentrated in middle-aged and older adults (>55 years), highlighting the need for vigilant safety monitoring in aging populations.
- Geography: The majority of reports originated from the United States (41.5%), followed by the United Kingdom, Japan, France, and Canada.
- Outcomes: Serious outcomes, including hospitalization (16.5%), death (3.0%), and disability (2.4%), were reported, emphasizing the public health importance of this analysis.
- Indications: BPH was the most common indication (41.3%), followed by dysuria, pollakiuria, and nephrolithiasis.
Adverse Event Signal Detection and Relevance to SDG 3
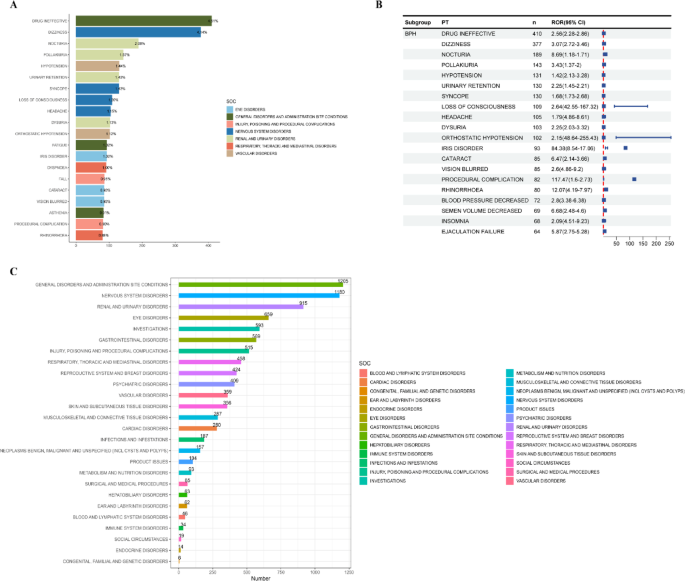
The analysis identified AEs across 27 System Organ Classes (SOCs). The most frequently reported SOCs were “General disorders and administration site conditions,” “Nervous system disorders,” and “Renal and urinary disorders.” At the Preferred Term (PT) level, the study confirmed several known AEs listed in official drug documentation, such as dizziness, syncope, and hypotension. Critically, this real-world analysis also uncovered new, previously undocumented signals, which is vital for ensuring medication safety (SDG 3):
- Hyperhidrosis (n=36)
- Cystitis (n=11)
- Panic reaction (n=5)
- Painful erection (n=3)
Temporal Analysis of Adverse Events
The median time to onset for AEs was 35 days. The analysis revealed a polarized distribution, with 46.74% of AEs occurring within the first month of treatment. This finding provides actionable information for clinicians to monitor patients closely during the initial phase of therapy, contributing to improved patient well-being as envisioned by SDG 3.
Subgroup Analysis: Addressing SDG 5 and SDG 10
Stratified analyses provided nuanced insights that support the goals of reducing inequalities.
- Gender Differences (SDG 5): The AE profile differed significantly between genders, reflecting different indications. In men treated for BPH, common AEs included vertigo and hypotension. In women, often treated for ureteral stones, reports of exposure during pregnancy, dosing errors, and unapproved indications were more prominent. This highlights the importance of gender-disaggregated data in healthcare, a key target of SDG 5 (Gender Equality).
- Age Stratification (SDG 10): The analysis of patients over 55 years of age showed results largely consistent with the overall population, confirming the relevance of the identified safety signals for this vulnerable group. This focus on specific age demographics supports SDG 10 (Reduced Inequalities) by ensuring health interventions are safe for all ages.
Discussion: Interpreting Findings in the Context of Global Health Goals
Real-World Evidence and Patient Safety (SDG 3)
This study demonstrates the value of real-world pharmacovigilance data in complementing evidence from clinical trials. The confirmation of known AEs and the detection of new signals provide a more complete safety profile for tamsulosin. This knowledge is fundamental to the rational and safe use of medication, a cornerstone of achieving universal health coverage and promoting well-being under SDG 3. The early onset of many AEs suggests that heightened clinical vigilance during the initiation of therapy could significantly improve patient outcomes.
Addressing Health Disparities (SDG 5 & SDG 10)
The distinct AE profiles observed between men and women, as well as across different age groups, underscore the necessity of personalized medicine and stratified safety monitoring. By generating evidence on how tamsulosin affects different populations, this research contributes to the goals of SDG 5 and SDG 10. The findings advocate for tailored clinical guidelines that account for gender- and age-specific risks, thereby reducing health inequalities.
Study Limitations and Future Directions
The analysis is subject to the inherent limitations of spontaneous reporting systems, including reporting bias and the inability to establish causality or calculate true incidence rates. The identified signals should be considered hypothesis-generating. Future prospective studies are required to validate these findings and more accurately assess the safety risks associated with tamsulosin, further strengthening health systems in pursuit of SDG 3.
Conclusion: Advancing Medication Safety for Global Well-being
This comprehensive pharmacovigilance analysis of the FAERS database provides significant real-world insights into the safety of tamsulosin. By identifying both common and rare adverse events, along with their temporal patterns and demographic variations, this report contributes valuable knowledge for clinical practice and regulatory oversight. Such research is fundamental to advancing the Sustainable Development Goal 3 agenda, ensuring that essential medicines are used safely and effectively to promote health and well-being for all populations.
Analysis of Sustainable Development Goals in the Article
1. Which SDGs are addressed or connected to the issues highlighted in the article?
-
SDG 3: Good Health and Well-being
The article is fundamentally centered on public health and safety, which is the core of SDG 3. It conducts a pharmacovigilance study to evaluate the safety of tamsulosin, a widely used medication. By identifying and analyzing adverse events (AEs), the study contributes to ensuring that treatments for non-communicable diseases like benign prostatic hyperplasia (BPH) are safe and effective. The focus on an aging population (“As the world ages, the incidence of BPH is also on the rise”) and gender-specific differences directly aligns with the goal of ensuring healthy lives and promoting well-being for all at all ages.
2. What specific targets under those SDGs can be identified based on the article’s content?
-
Target 3.4: Reduce mortality from non-communicable diseases and promote mental health
The article addresses this target by focusing on the safe management of BPH, a prevalent non-communicable disease in aging men. The study’s aim to identify “new and significant adverse reactions” helps improve treatment protocols, which can reduce complications and promote better health outcomes. Furthermore, the identification of AEs like “panic reaction” directly touches upon the mental health aspect of this target.
-
Target 3.8: Achieve universal health coverage, including access to quality and safe essential medicines
Tamsulosin is described as “among the most commonly prescribed medications” for its indications, making it an essential medicine for many. This study’s evaluation of its safety using the FAERS database is a critical component of ensuring the quality and safety of essential healthcare services and medicines. The analysis of over 10,071 reports aims to provide a “foundation for the rational clinical use of the drug,” directly supporting the goal of providing safe and effective medicines for all.
-
Target 3.d: Strengthen the capacity for early warning, risk reduction, and management of national and global health risks
The entire study is an example of this target in action. It utilizes the FDA Adverse Event Reporting System (FAERS), a key tool for “postmarket surveillance” and “early warning” of health risks associated with drugs. The methodology, described as a “retrospective pharmacovigilance analysis,” is a form of risk management. The identification of “unexpected and rarely reported signals” such as hyperhidrosis and cystitis demonstrates the system’s capacity to detect potential new health risks, which is crucial for public safety.
3. Are there any indicators mentioned or implied in the article that can be used to measure progress towards the identified targets?
-
Indicators for Target 3.4
The article provides data that can serve as proxy indicators for health outcomes and disease management. These include:
- Prevalence of non-communicable diseases: The article cites that “approximately 50–60% of men over 50 experience symptoms related to BPH, with prevalence reaching up to 80% in men over 70.”
- Serious health outcomes from treatment: The study quantifies serious adverse events, including “hospitalization, death, and disability events, which occurred in 1659 (16.5%), 302 (3.0%), and 243 (2.4%) cases, respectively.” These numbers indicate the health burden associated with medication side effects.
-
Indicators for Target 3.8
Progress towards providing safe medicines can be measured by monitoring adverse events. The article uses several quantitative indicators:
- Number of adverse event reports: A total of “10,071 reports listing tamsulosin as the primary suspected drug were identified.”
- Frequency of specific AEs: The article lists the top 20 most frequent AEs, such as dizziness, hypotension, and falls, providing a measure of the most common safety concerns.
- Statistical signal strength of AEs: The use of metrics like Reporting Odds Ratio (ROR), Proportional Reporting Ratio (PRR), and Empirical Bayesian Geometric Mean (EBGM) are direct indicators used to quantify the strength of association between the drug and an adverse event.
-
Indicators for Target 3.d
The capacity for health risk management is demonstrated through the systems and outcomes described in the article:
- Functioning of a pharmacovigilance system: The use of the FAERS database, which has been “monitoring and documenting drugs since 2004,” is an indicator of a robust national system for health risk management.
- Identification of new safety signals: The detection of “new signals of previously unreported hyperhidrosis, cystitis, panic reaction, and painful erection” serves as a direct indicator of the system’s effectiveness in providing early warnings.
- Time-to-onset (TTO) analysis: The finding that “46.74% of AEs occurred within the first month” is a crucial indicator for risk management, helping clinicians know when to monitor patients most closely.
4. Table of SDGs, Targets, and Indicators
| SDGs | Targets | Indicators |
|---|---|---|
| SDG 3: Good Health and Well-being | 3.4: Reduce mortality from non-communicable diseases and promote mental health. |
|
| SDG 3: Good Health and Well-being | 3.8: Achieve universal health coverage, including access to quality and safe essential medicines. |
|
| SDG 3: Good Health and Well-being | 3.d: Strengthen the capacity for early warning, risk reduction, and management of health risks. |
|
Source: nature.com